We accelerate the entry of medical innovations into the market
We connect science, data, regulation and market to deliver products with compliance and high ROI impact.



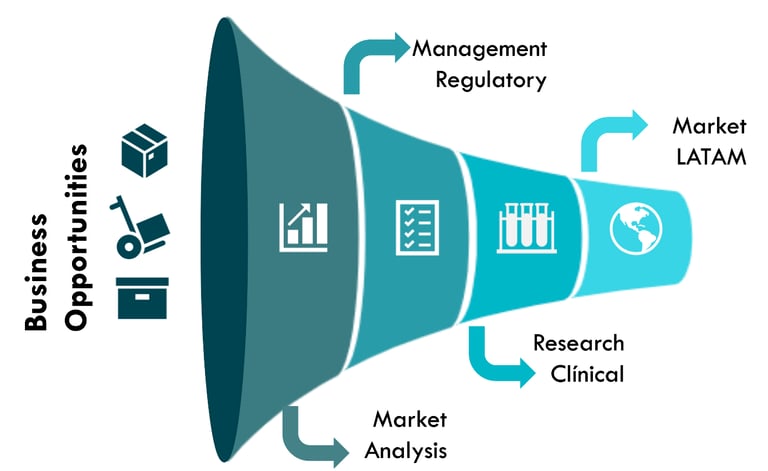
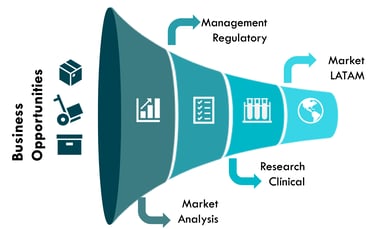
Accelerating access to medical innovations in the Latin American market. We offer comprehensive and strategic solutions that integrate:
Market Research
Risk Assessment
Conversion Rate Optimization (CRO)
Regulatory Analysis
Commercialization
Ensuring agility, security, and maximum impact.
We work under international standards of quality and ethics, with a focus on measurable ROI and tangible results.
We are your gateway to Latin America.
Our key services
Product Analysis and Diagnosis:
We evaluate market potential, trends, and regulatory feasibility to optimize the launch strategy.
End-to-End Regulatory Management:
We coordinate clinical trials with comprehensive monitoring, from design to completion, ensuring compliance and reduced timelines.
Clinical Research Organization (CRO)
Clinical Research Organization (CRO) Management End-to-End Regulatory Management: Distribution Channel Development We manage the entire sanitary registration cycle with authorities such as COFEPRIS, ANVISA, and INVIMA, achieving over 75% efficiency in processing applications.
Opening of Distribution Channels
We develop B2B and B2C networks, leveraging business intelligence and import/export strategies for effective expansion.
At Vertex CRO we connect science and health with the world


