We accelerate the entry of medical innovations into the market
We connect science, data, regulation and market to deliver products with compliance and high ROI impact.




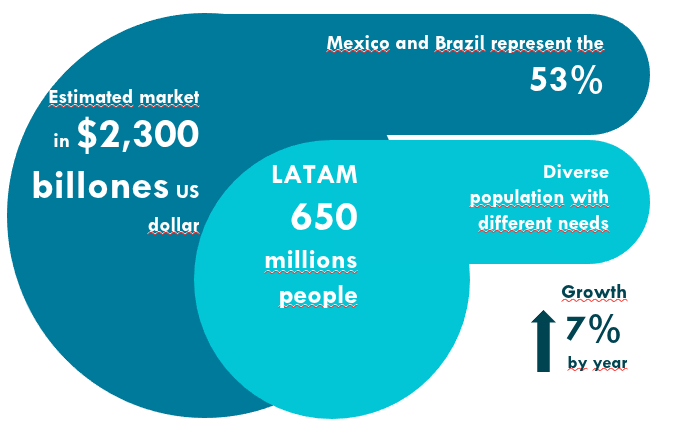
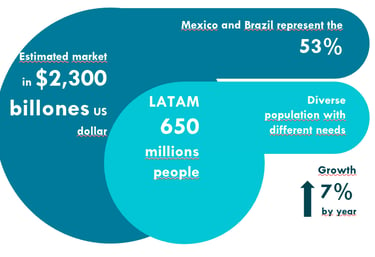
The Latin American Life Sciences market is no longer an option—it is a global strategic priority. The data validates the urgency: the region represents an estimated value of $+ USD 2.3 Trillion and projects a robust 7% annual growth.
Our expertise is laser-focused on guiding this massive healthcare investment.
Mexico and Brazil alone concentrate over 53% of the regional market, providing strategic access to over 650 million inhabitants.
At Vertex CRO, we leverage Mexico’s Accelerated Regulatory Approval (Fast Track) pathway to drastically reduce your time-to-market. We transform regulatory complexity into your competitive edge, ensuring your medical device launch or innovative drug expansion across LATAM achieves maximum ROI.
VERTEX is the ideal partner for companies seeking to expand with strategy, agility, certainty and impact, focused on results.
Why LATAM, why Vertex
The health sector in Latin America is experiencing rapid growth:
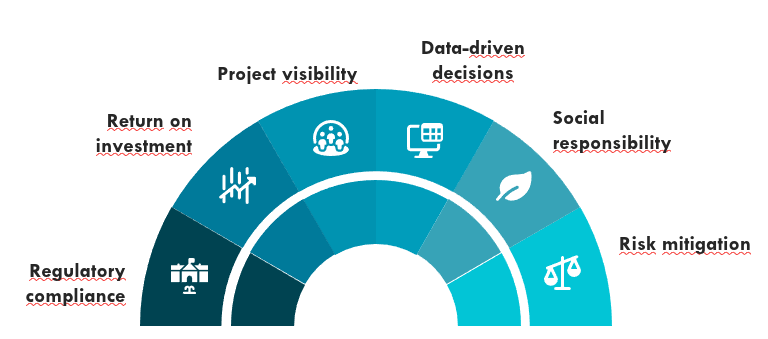
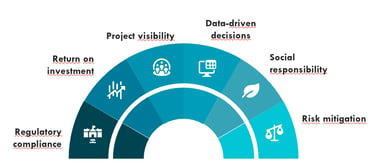
Key benefits
We deliver Clinical Research and Regulatory Compliance driven by efficiency and precision.
Our methodology focuses on reducing regulatory timelines through proven Fast Track processes, giving you complete project visibility for absolute control.
We guarantee sound data-driven decisions and proactively mitigate risk, resulting in a significant reduction in operational risk and maximizing your Return on Investment (ROI) during your pharmaceutical expansion across LATAM.